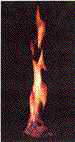METHANE AND METHANE HYDRATES, SECTION 1
At such [peaceful] times, under an abated
sun; afloat all day upon smooth, slow heaving swells; seated in
his boat, light as a birch canoe; and so sociably mixing with
the soft waves themselves, that like hearthstone cats they purr
against the gunwale; these are the times of dreamy quietude, when
beholding the tranquil beauty and brilliancy of the ocean's skin,
one forgets the tiger heart that pants beneath it...
Melville, Moby Dick, Chapter CXIII

Lifting the skin of the sea. Dali at the age of six, when
he thought he was a girl, lifting the skin of the water to see
a dog sleeping in the shade of the sea (1950
oil painting by Salvador Dali). The ocean
and the atmosphere constantly exchange gases and small particles
(of things like salt), maintaining a rough equilibrium. The ocean
also has a great heat capacity, that is, a great ability to hold
heat. As the atmosphere heats up because of global warming, it
transfers much of that heat to the ocean. Though the ocean absorbs
that heat slowly, it will also (eventually) lose that heat very
slowly, insuring that the heat we now inject into the atmosphere
via carbon dioxide emissions will be around for a very long time.
In November of the year 2000, the Ocean
Selector, a fishing trawler operating in the Pacific off the west
coast of Vancouver Island, Canada, hauled up a very unusual catch.
Along with the fish it obtained from a seafloor depth of about
800 meters (about half a mile) were numerous chunks of a white,
frigid snowball-like substance. There was, in fact, a huge amount
of the compacted but lightweight substance: perhaps 1000 kilograms
(more than a ton), possibly more than had ever been netted before.
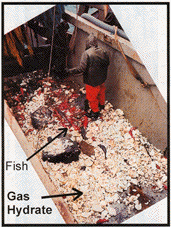 |
The catch of the Ocean Selector.
(Photo: Spence, 2001)
|
Most of the fishermen were perplexed
about their unexpected catch, but a fisheries observer who happened
to be on board suggested it might be something he had never personally
seen before, something called methane hydrate, the mysterious
"ice that burns" (Spence, 2001).
Methane is a tasteless, odorless, colorless
gas. Along with tiny traces of other gases, it is the primary,
almost exclusive, constituent of the "natural gas" which
fuels many gas stoves and home heaters. Because it is a dangerous
gas, potentially asphyxiating, flammable, and explosive, an unpleasant
odor (that of methanethiol, or methyl mercaptan, CH¸3SH)
is added to domestically used natural gas so that we can detect
it by smell. In coal mines, however, methane provides no such
warning; hence, the "miners' canary" was once used to
give early notice of methane's presence, because canaries are
more sensitive to methane than people. In spite of such precautions,
many coal miners are nevertheless still lost every year in methane
explosions.
Each molecule of methane is composed
of four atoms of hydrogen held together by one atom of carbon
(the chemical formula is CH¸4). With such a composition
(hydrogen and carbon), methane is referred to as a hydrocarbon.
Methane is a major component of the atmospheres of the "gas
giants" of our solar system, Jupiter, Saturn, Uranus, and
Neptune, and probably was a major constituent of the early atmosphere
of Earth itself.
But methane is fairly active chemically
and readily reacts in the atmosphere, and, to a lesser extent,
in seawater, in a series of complex reactions whose end products
are carbon dioxide and water. Once
Earth's marine photosynthesizing organisms became plentiful in
surface waters about two and a half billion years ago, producing
a low level of free oxygen in our atmosphere and in the surface
layer of the ocean, most free methane would have combined with
that oxygen and disappeared. Because of the over 20% abundance
of oxygen in our current atmosphere, there is little free methane
around, though its quantity is slowly increasing. The presence
of free oxygen, however, limits the lifespan of free methane in
the atmosphere and most of the ocean to less than ten years.
The primordial methane of Earth's ancient
atmosphere having long ago been oxidized, the subsequent atmospheric
methane, ancient as well as modern, has been produced thermally
or biologically. (The methane produced by these different processes
is therefore referred to as thermogenic or biogenic methane.)
Methane is produced thermally by the decomposition of organic
matter by heat from the interior of the Earth. Most of the planet's
methane, however, is probably produced biologically. Organisms
called methanogens ("methane-makers") discard methane
as a waste product of their metabolic activities, which take place
in environments free from, or protected from, oxygen.
Archaea and Methanogens
The most important location for the formation
of methane is in the sediments of the ocean floor. Within the
sediments, in addition to the lithic (rock) particles, the dead
organisms -- mostly microscopic -- which have rained down through
the water column or been carried from the land, and various sorts
of organic debris, are a multitude of living organisms including
burrowing worms and mollusks (at shallow depths), and an awesome
array of microorganisms (throughout the sediments: D'Hondt, 2004).
Many of these microorganisms belong to the familiar category bacteria,
but others are part of a group whose existence was not even suspected
just a few decades ago. These microorganisms are Archaea.
|
Archaea
Archaea is the name of the classification. The word archaea,
with the "a" in lower case, is the plural form and
refers to a number (two or more) of species or individual organisms
within that class. (The term archaeans is also sometimes used
to denote the plural form.) The word archaeon is the singular
form, and refers to a single species or individual. Similarly,
Eubacteria is the classification name; bacteria refers to more
than one species or individuals; bacterium refers to a single
individual or species. One does not often run across a reference
to single individuals ( of either bacteria or archaea), however,
so the singular and plural forms typically refer to species.
The curious singular and plural forms (archaeon/archaea and bacterium/bacteria)
derive from the fact that Greek and Latin words, respectively,
are employed.
The term Archaea for
these organisms, however, should not be confused with the term
used for one of the oldest geologic time periods, the Archean
Eon, often shortened simply to the Archean. (In some geologic
time scales, the Archean includes the Hadean [Eon], the oldest
time period.) Archaea refers to the group of organisms, Archean
refers to the time period. Both terms derive from the Greek word
'archeo,' meaning primitive.
|
It used to be thought that the basic
distinction in the living world was between bacteria, which are
small, unicellular, and relatively simple (though indeed the simplicity
is only relative, all living organisms being quite complex), and
larger, often multicellular organisms. The bacteria are part of
a group called the prokaryotes (in fact, they used to be considered
the only members of that group), whose DNA is not enclosed in
a nucleus.
All other organisms are eukaryotes, which
do enclose their DNA with a nucleus, and which include all the
multicellular organisms we routinely encounter in daily life,
such as plants, fungi, and animals. The eukaryotes also include
many creatures we do not encounter daily, organisms of the unicellular
variety called protoctists (sometimes referred to as protists,
but this term may also refer to any single-celled organism, even
those which are not eukaryotes). Eukaryotic cells generally are
considerably larger than prokaryotes, and they contain small but
distinct organs, referred to as organelles (chloroplasts, which
contain chlorophyll, and mitochondria, which provide cell energy,
are the most obvious), which prokaryotes lack. (Some bacteria
have now been found to contain an organelle also present in single-celled
eukaryotes; see Seufferheld, 2003).
Microbiologists Lynn Margulis and Karlene
Schwartz, noting the one large prokaryote group, the bacteria,
and the four large groups of the eukaryotes proposed that all
living things comprised five "kingdoms," the highest
level of biological classification. Because the prokaryotes were
quite distinct from the eukaryotes, they illustrated their proposal
as a human hand, with the bacteria set off as the thumb, and the
eukaryote groups as the fingers. Despite considerable variation
in size, eukaryotic cells are typically about ten times the linear
dimensions of prokaryotic cells, and their volumes are about 1000
times greater.
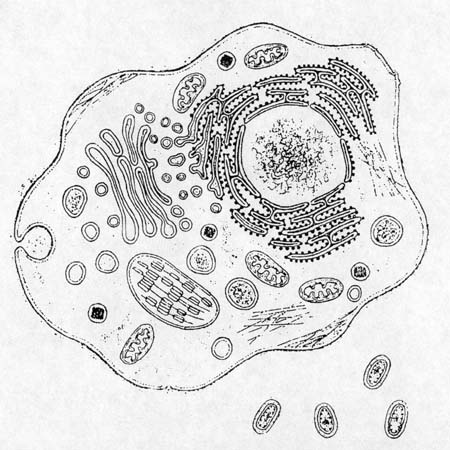
Eukaryote and prokaryotes, relative sizes. (Diagram from Gross, 1996,
p. 134.)
In the '60's and 70's, however, some
new organisms were discovered which, though unusual, were relegated
to the classification bacteria largely because they were unicellular,
small, did not have their DNA bound up in a nucleus, and, most
importantly, because there was apparently no other biological
category into which they might be fit. These unusual organisms
were often found in environmental conditions considered extremely
hostile to life, places of quite high temperature, salinity or
acidity. Later, these inhospitable habitats would earn their inhabitants
the name "extremophiles," that is, lovers of extreme
(conditions).
With the development of new analytical
methods by molecular biologists, the actual makeup of organisms
at the molecular level -- a vastly smaller level than that of
the cell -- began to be explored. The determination of the structure
of DNA -- the famous double helix -- was one of the first triumphs
of these new methods. By the mid 70's, molecular biology had advanced
to the point that differences between the molecules of various
organisms were being employed to examine the evolutionary relationships
between them, just as DNA testing of human beings is today used
to determine relations between individuals and groups.
In 1977, biologists using RNA from ribosomes,
the protein factories of cells, to examine numerous species of
bacteria made a startling discovery: that a few of the bacteria
were vastly different, on a molecular level, than the many thought
to be their biological cousins. In fact, biologists Carl Woese
and George Fox, decided these organisms were so unlike other bacteria
that they couldn't really be considered bacteria at all, but had
to be assigned their own special classification, first called
Archaebacteria, then changed and shortened to Archaea to make
clear that they were entirely distinct living things.
The distinctiveness of the Archaea required
a major revision in thinking about biological classification.
No longer could the highest division of living things be considered
the kingdom. Instead, while preserving the classification kingdom
for the next lowest subdivisions, a new and higher division was
created: the domain. Living things would be divided into three
domains: Eubacteria, Archaea, and Eukaryota, the eukaryotes.
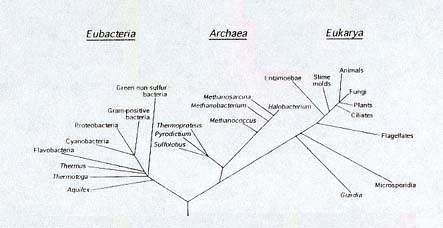 The three branches (domains)
of life. Eubacteria and Archaea
are prokaryotes, relatively simple, single-celled organisms. Eukaryotes
(Eukaryota) possess complex cells; some are single-celled. All
multicellular organisms, including animals, plants, and fungi,
are eukaryotes. The line lengths are based on studies of the genetic
information of the organisms; they indicate how closely the organisms
are related. Note how closely animals, plants, and fungi (upper
right) are related, and how far removed they are from many other
organisms. (Howland, 2000, Figure 2.2, p. 29)
The three branches (domains)
of life. Eubacteria and Archaea
are prokaryotes, relatively simple, single-celled organisms. Eukaryotes
(Eukaryota) possess complex cells; some are single-celled. All
multicellular organisms, including animals, plants, and fungi,
are eukaryotes. The line lengths are based on studies of the genetic
information of the organisms; they indicate how closely the organisms
are related. Note how closely animals, plants, and fungi (upper
right) are related, and how far removed they are from many other
organisms. (Howland, 2000, Figure 2.2, p. 29)
Not having their DNA enclosed in a nucleus,
archaea are prokaryotes like bacteria. And there are other similarities:
most prokaryotic DNA is found in the form of a single chromosome,
a single large unbroken loop, sometimes together with smaller
loops called plasmids. By contrast, eukaryotic DNA, enclosed in
a nucleus, is divided into many chromosomes, and the chromosomes
take the form of short spaghetti-like strands.
The difference is a critical one, one
which goes far beyond the apparent shape and number: the short
strands of the eukaryotic chromosomes have at their ends many
copies of special units of DNA called telomeres, which have been
likened to the plastic tips of shoelaces. Each time the cell reproduces,
another unit is removed. Eventually, when all the telomere units
are gone, the remaining DNA becomes unstable and the cell dies.
Consequently eukaryotic cells have limited lifespans, programmed
into their DNA from their inception. By contrast, the single loops
of prokaryotic DNA, being loops, lack such telomeres, and thus
are not programmed for eventual demise. Prokaryotic cells can
divide and divide (a process called binary fission) and, in essence,
live forever, while most eukaryotic cells, like the organisms
of which they are a part, eventually must die. Sexual reproduction,
by creating new individuals, is the eukaryotic organisms' hedge
against oblivion.
One major difference between archaea
and other living things, however, lies in the way they construct
their outer cell membranes. Archaea employ ether links between
the organic components of their cell membranes. Most other organisms
use ester links. Ether links are chemically more stable, and such
links may have helped archaea survive in the hostile conditions
of early Earth. In addition, while all organisms have chains of
fatty acids as essential components of their cell membranes, those
chains are usually straight. In archaea they are branched, or
"isoprenoid" (Howland, 2000, p. 74-79). Both the ether
links and the isoprenoid configuration of the fatty acid chains
provide biochemical indicators that archaea are present.
This biochemical evidence turns out to
be quite important where other methods for detecting archaea are
unsuccessful. Microorganisms generally cannot be identified where
they are found, because they are too few and too small to be seen
without substantial magnification. Indeed, one archaeal group,
the Korarchaeota, has only been detected by its biochemical traces.
No korarchaeon has ever been seen. But this is not surprising:
microorganisms are notoriously difficult to isolate and identify,
and the few that have been are generally those that fare well
in laboratory conditions.
Only a tiny percentage (perhaps only
1%) of microorganisms can be cultured, that is, grown in the laboratory
in significant numbers, outside of their natural habitats. Since
the number of a particular microorganism in a given sample may
be quite small, the ability to raise them in significant quantities
is extremely important. Thus those microorganisms which cannot
be cultured generally escape our attention. To increase the likelihood
that particular microorganisms may be cultured and thereby identified,
scientists attempt to replicate the precise conditions in which
a sample was taken. If the sample were taken from one of the hot
pools in Yellowstone National Park, for example, scientists will
try to recreate the heat, acidity, dissolved gas and nutrient
conditions found in the original pool, both during the transfer
process and in the lab.
Nonetheless, the effort is often unsuccessful.
A major reason for culture failure, it turns out, is that many
microorganisms do not solely depend on the relatively large scale
conditions mentioned above: heat, acidity, dissolved gas, and
nutrients. Often they depend on very specific "local"
conditions, which, in the case of microscopic creatures, can be
very local indeed. Furthermore, the congeniality of these local
conditions frequently requires close proximity to other particular
microorganisms, which help provide the very specific conditions
the organism of interest requires to thrive or even survive. It
is not surprising, therefore, that recent efforts that include
taking slabs of sediment rather than the small samples previously
obtained should have allowed richer cultures to be grown.
Ecosystems comprised of different kinds
of microorganisms living together are known as consortia. Consortia
are a microscopic version of the ecological communities with which
we are more familiar, like plant-herbivore-predator communities.
Although most relationships between organisms in these consortia
are only beginning to be investigated and understood, some organisms
clearly provide products which are useful to other consortia members,
and, in some cases, there is likely to be the kind of mutual exchange
known as symbiosis. (In the case of microorganisms, symbiosis
is known by the term syntrophy, literally, "eating together.")
Symbiotic processes are found throughout
the natural world. In its broadest sense, symbiosis is much more
common than many biology texts indicate. Lichens, for example,
are typical textbook symbionts: they represent the simple pairing
of an alga and a fungus, an arrangement that allows the lichen
to live on the surface of rocks, frequently in quite dry, frigid,
or otherwise inhospitable conditions. Surprisingly, however, as
many as 20% of fungal species may engage in these partnerships.
Much more important to us is the symbiotic
relationship between terrestrial plants and the fungi known as
mycorrhizae (literally, "root fungus"). This is a relationship
which began perhaps 450-500 million years ago, and was essential
for the evolution and spread of plants onto the land. Mycorrhizal
fungi are microorganisms which live on the roots of plants and
allow plants to take up water and minerals from the soil. Quite
recently, fungi have been found to inhabit almost all cells of
photosynthetic land plants, in addition to their roots. Without
these symbiotic organisms, we wouldn't have most of our food supply.
The digestive systems of cows, sheep,
goats, giraffes, antelopes, buffalo, camels, and others -- those
animals known as ruminants -- also house symbiotic organisms:
methanogens. These methanogens live off the grass or leaves that
the ruminants consume, and the ruminants in turn obtain much of
their protein from digesting their store of methanogens and other
gut microorganisms (Howland, 2000, p. 91). The by-product of this
arrangement is methane, of use to neither cow (for example) nor
methanogen, but released into the atmosphere as cow burps. A similar
relationship between termites and methanogens allows termites
to consume wood, again releasing methane as a waste product. Much
atmospheric methane thus owes it origin to ruminants and termites.
The methanogens in which we are interested,
however, live in the anoxic sediments of the ocean floor. These
sediments, which can accumulate to depths of many hundreds of
meters (well over a thousand feet), lack oxygen except in their
topmost few centimeters (an inch or so). (Some oxygen, apparently
from water flowing through bedrock, does seep into the base of
the sediments [D'Hondt, 2004], but it is unimportant for our purposes.)
In the sediments, many microorganisms
consume the organic detritus which has rained down through the
water column. This process of consumption both depletes the remaining
oxygen and releases carbon dioxide. That carbon dioxide, and other
carbon sources, provide the carbon from which the methanogens
manufacture methane. Other organisms contribute hydrogen (Gross,
1996), though hydrogen is also produced by inorganic means, through
the interaction of ocean floor basalt and water (Stevens and McKinley,
1995), and even by a certain kind of radioactive decay (alpha
particles -- helium nuclei -- colliding with water molecules,
create hydrogen peroxide, oxygen, and hydrogen gas; Fields, 2003).
The Archaean domain is comprised of three
different groups, based on where they live and what they do to
make a living. (These groupings are similar to classifying people
according to their home neighborhoods and occupations, and do
not necessarily reflect how closely they may be related.) One
group is the thermophiles, which, as their name implies, prefer
hot conditions. These hot conditions are found in two situations,
either where extremely hot water comes pouring out of the earth
under the sea, at the sites of black smokers, white smokers, or
hot seeps, or in the hot pools and geyser basins that are found
in areas of volcanic activity, as at Yellowstone.
In these situations, water temperatures
can exceed the boiling point of water (100°C; 212°F);
in fact, the water that comes out of black smokers can be many
hundreds of degrees hot. Nonetheless, some thermophiles, called
hyperthermophiles, can survive temperatures above 100°C, because
they exist at ocean depths where the pressure is so great (100
to 500 times that of the surface of the earth: Gross, 1996) that
the water cannot become steam. Other thermophiles prefer somewhat
cooler temperatures, in the 85°C range.
A second group of archaea are the halophiles
("salt-lovers"). These creatures prefer extremely saline
conditions such as in deserts where high evaporation rates prevail.
During wet seasons, thunderstorms, or in flash floods, rainwater
washes down sediments and soluble materials from surrounding mountains
into desert basin areas. There, without any outlet, the water
evaporates under the unrelenting sun, eventually leaving behind
only a dry playa. But before the basin gets to that stage, if
it ever does, the salt content rises. The Dead Sea, between Israel
and Jordan, is one well-known example, and the Utah's Great Salt
Lake is another.
Water in the Great Salt Lake is about
25% salt, making it easy to float in (in fact, making it impossible
not to float in), but unpleasant for getting in one's eyes. Halophiles
don't find it unpleasant at all. While other microorganisms would
find their water sucked from their bodies like prunes by the salinity,
the halophiles protect themselves by incorporating a higher concentration
of salt than is found in their environment. One curious feature
of the Great Salt Lake, however, is that the water is pink. That
pinkness is the consequence of the archaean halophiles which enjoy
its salinity. Despite their extreme situations, they actually
engage in a type of photosynthesis, though not using chlorophyll.
It is the purple color of the pigment they employ for photosynthesis
that makes the water pink.
The third group of archaea are the methanogens.
The name derives from the fact that methanogens make (generate)
methane, just as hydrogen, combined with oxygen (that is, when
burned), makes water (hydro in Greek). The methanogens are divided
into five subclassifications (called "orders"; see BOX:
Biological classification, in Permian World section), Methanococcales
(about 9 species), Methanobacteriales (about 25 species), Methanosarcinales
(about 19 species), Methanomicrobiales (about 22 species), and
Methanopyrales (1 species; Madigan, 2003). (The methano- indicates
that these archaea are methanogens; the second part of the name
refers to the organism's shape: coccus = sphere, bacteria = rod,
sarcina = cubic, and microbiales = extremely small, round, flat,
onion-flavored rolls: bialies are a breakfast favorite of many
New Yorkers. The last order, Methanopyrales, derives its name
not from the shape of its single species, methanopyrus, but rather
from the ecological conditions in which that species lives. "Pyro,"
as in pyromaniac, comes from the Greek word for fire; methanopyrus
lives in hot springs at extremely hot temperatures.)
Interestingly, it is clear that some
methanogens can actually turn around and consume methane under
particular conditions (Hinrichs, 1999; Hallam, 2004). These archaea,
like some bacteria that also can consume methane, are referred
to as methanotrophs (literally, "methane-eaters"). Some
archaea, however, may be exclusively methanotrophic, and could
therefore constitute a fourth division of the Archaea (Hinrichs,
1999).
|
Archaea Classification
Formally, the archaean domain, Archaea, is divided into three
groups. These groupings are based on the actual relatedness of
the organisms, rather than how they make their livings. There
is some disagreement as to the classification level (scientists
use the term "taxonomic" level to refer to classification
level; see BOX: Biological classification, in Permian World section)
level in which these groups should be placed. The next lower
level after domain is kingdom, and the one below that is the
phylum, and some scientists have placed the basic archaeal division
at the kingdom level, while others consider the main groups as
at the phylum level. The classification level reflects how closely
the groups are related: whether they are near or distant "cousins."
But while the classification
level issue may be unresolved, there is general agreement on
the main archaean groups. They are the Euryarchaeota, which contains
the methanogens and halophiles and some of the thermophiles,
and the Crenarchaeota, to which many of the thermophiles belong.
A third group, the Korarchaeota, is known only from their organic
residues rather than from the organisms themselves. Although
the Korarchaeota may constitute a special side branch of the
Crenarchaeota, there is no question that its members -- yet to
be isolated -- are quite distinct from other archaea. Their RNA
tells us so. In fact, it is the RNA differences among the archaea
which has allowed biologists to distinguish the relations and
divisions among the various groups. The recent discovery of a
new archaeal organism, Nanoarchaeum (Huber, 2002), has led to
a proposal that an additional group of archaea may exist, based,
however, on the microbe's distinctive DNA.
As scientists have continued
their investigations of the Archaea, they have made some stunning
discoveries. Unknown and unsuspected until just two decades ago,
archaeans turn out to be major, if not dominant, constituents
of the biota of oceans and soils, and significant contributors
to essential biochemical processes therein. In both oceans and
soils, archaeans oxidize ammonia, a critical step in the production
of biologically usable nitrogen compounds (a process called nitrogen
fixation). It used to be thought that this process was carried
on solely by bacteria, but it is now known that archaea oxidize
tens to hundreds -- and, in some situations, perhaps thousands
-- of times more ammonia than bacteria (Leininger, 2006; Wuchter,
2006). "Higher" plants, like photosynthesizing land
plants, cannot accomplish this task on their own, and are completely
dependent on microbes to provide them with this essential nutrient.
In the oceans, it has been discovered, Crenarcheota "are
the most abundant single group of prokaryotes" (Wuchter,
2006).
|
Living things make their livings in two
basic ways: they either make and consume their own food (a process
called autotrophy), or they consume other living things, or the
organics they create (a process called heterotrophy. We are heterotrophs,
and as such rely on other living things for our food supply, virtually
all of which comes directly or indirectly from plants (with the
minor exceptions of fungi and seaweed -- neither is a true plant,
incidentally -- which provide rather little nutritional value).
The most familiar kind of autotrophy
(photoautotrophy) is photosynthesis, which most commonly employs
chlorophyll to facilitate the manufacture of a basic sugar unit
(CH¸2O, six of which are assembled to form glucose, a simple
sugar: C¸6H¸12O¸6) from the raw materials water
and carbon dioxide. The chemical equation for this process is:
H¸2O +
CO¸2
Æ
CH¸2O
+ O¸2
(water) + (carbon dioxide) (yields) (basic sugar unit) + (oxygen)
It is important to note that the useful
product of this process is the basic sugar unit, and not the oxygen,
most of which is simply a waste product (though some is used for
cellular respiration) and is dumped.
Photosynthesis is conducted in today's
world by four main groups of organisms: cyanobacteria, two other
kinds of marine phytoplankton, and the plant kingdom. Phytoplankton
include all free-floating oceanic microorganisms that engage in
photosynthesis, and therefore include cyanobacteria and two main
groups of eukaryotic organisms: the diatoms and coccolithophorids.
On land, green plants constitute the fourth group of photosynthesizers.
All of these four groups with the exception of the coccolithophorids,
also referred to as coccolithophores or coccoliths, were around
in the Permian.
Cyanobacteria used to be called blue-green
algae, because they used to be considered plants. But it is now
clear that they belong to the classification Eubacteria, and are
only very distantly related either to the photosynthesizing eukaryotes
of the phytoplankton (the diatoms and coccolithophorids) or to
the green plants of our ordinary experience. Despite that distant
relationship, however, there is a very important connection between
cyanobacteria and all other photosynthesizing eukaryotes, including
green plants.
All eukaryotic photosynthesizers are
able to photosynthesize because they contain chlorophyll, enclosed
in cellular organelles called chloroplasts. In the 1960s the microbiologist
Lynn Margulis recognized that many of the organelles of eukaryotes
were actually former symbionts which more than a billion years
ago lived independently of the eukaryotes in which they are now
found. (They are referred to as endosymbionts because they are
inside rather than outside the cell). It is now generally accepted
that the original cyanobacterial ancestor of the chloroplast was
captured during the early evolution of the eukaryotic cell, some
billion and a half years ago.
There is a second kind of autotrophy
which is far less familiar. This kind is labelled chemoautotrophy
because it relies on chemical processes rather than light for
the energy needed for food production. Instead of dumping oxygen,
these organisms dump other metabolic waste products. Methanogens,
the ones with which we are most concerned, dump methane. Although
numerous organic molecules, including acetate, formate, and methyl
alcohol, can be used as the source of carbon, the simplest methanogenesis
reaction employs carbon dioxide and hydrogen:
CO¸2
+
4H¸2
Æ CH¸4
+ 2H¸2O
(carbon dioxide) + (hydrogen) (yields) (methane) + (water)
The main product of this reaction for
the methanogen is not methane, which is waste (at least for the
methanogen), or water, which is obviously plentiful in the watery
environments in which methanogens are found, but energy. The reaction
releases a certain amount of energy which the organism then puts
to use in assembling the organic molecules it needs for its existence.
A good deal of our atmosphere's methane
comes from the activity of methanogens in wetlands, especially
in colder regions, and in the rice paddies of warm climates. Although
methanogens reside in numerous exotic environments -- the guts
of cows and termites, swamps, northern peatlands, rice paddies,
Yellowstone hot springs, and undersea vent communities -- those
of greatest interest to us here live buried deep in the ocean-bottom
sediments.
A huge quantity of methane is produced
in the sediments of the ocean floor. When marine organisms and
microorganisms die, their corpses rain slowly down to the bottom
of the ocean. In the process, they are mixed with silty (fine-grained),
sandy or pebbly sediments washed into the ocean by rivers. The
sediments and the organic matter come to rest on the ocean floor,
to be buried by further sedimentation. Because most marine organisms
live relatively close to shore and because most of the sediment
washing off the continents is carried only a short distance, a
considerable portion of the organic debris and mud winds up on
those parts of the ocean which are shallow and close to the continents.
These areas are called the continental
margins. The part of the continental margin that is shallowest,
flattest, and closest to shore is called the continental shelf.
The continental shelf drops down to an average depth of about
130 meters (400 feet). At about this depth, there is a "shelf
break," and the ocean floor drops more steeply down what
is referred to as the continental slope, to the great depths of
the oceans' abyssal plains, the deepest and flattest parts of
the oceans.
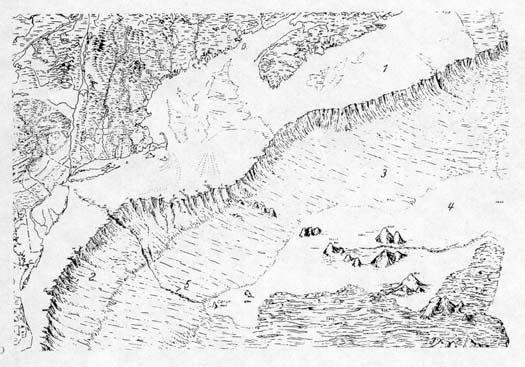 |
| The ocean floor off the
Middle Atlantic states, New England, and eastern Canada. The
number 1 indicates the continental shelf; 2, the continental
slope; 3, the continental rise, 4, the abyssal plain, and 5,
a submarine canyon cut by water from the Hudson River. Seamounts
rise from the abyssal plain. Map from Siebold and Berger, 1982. |
The dead organisms in the continental
margin muds mostly decay in conditions that exclude the presence
of free oxygen; these are called anoxic environments. Though there
is often a good deal of oxygen in deep ocean water, the oxygen
is rapidly depleted in the upper few centimeters (an inch or two)
of sediment by microorganisms which employ that oxygen in the
process of consuming organic debris. With the extreme oceanic
cold at these depths, however, the process of organic decay is
quite slow.
Below the oxic zone, those top few centimeters
of sediment where oxygen is still present, the organic decay becomes
even slower and much less efficient. This is because the process
of anaerobic decay -- that is, the process of decay by organisms
that do not use free oxygen -- is vastly slower than that by aerobic
(oxygen using) organisms. But even in sediments hundreds of meters
below the seafloor, decay is still going on. At such depths, in
fact, the chill imparted by the overlying frigid ocean water begins
to give way to the warmth generated from within the Earth. Because
the efficiency of metabolism at these temperatures increases with
warmth, decay may become more efficient, as long as chemical energy
sources like hydrogen, sulfate, or various organic compounds such
as acetate are available. It is in this region of sediment well
below the seafloor that the methane-makers thrive, and the production
of methane takes place.
Being a lightweight gas, the methane
produced by the methanogens rises. Though -- as we shall see --
most methane never makes it so far, some eventually does reach
the top five or so centimeters (about two inches) of the sediment.
|
The Seafloor
Though the term "seafloor"
is regularly used herein, the seafloor in most parts of the ocean
is not a simple, solid surface. It is not composed of rock, but
of accumulations of sediment that have settled there from the
time that the ocean basin originated. While the deepest sediments
have been compressed by the weight of overlying sediments and
sometimes turned to rock, the shallower sediments are less and
less consolidated with decreasing age and depth. Approaching
the surface, the sediments are the consistency of mud, though
in places they are mostly water. Higher still, the sediment has
such a high water content that it is like a soup or slurry. Near
its contact with the ocean itself, the uppermost layer is nothing
more than muddy water. Because of the lack of a solid surface,
oceanographers often prefer the term "sediment-water interface"
instead of seafloor.
Because much sediment originates on continents, it is generally
more plentiful close to the continents, and particularly at the
mouths of large rivers, creating their deltas. Rivers like the
Nile, Mississippi, Niger, Chang Jiang (Yangtze), Ob, Indus, Ganges
and Brahmaputra all have substantial deltas, but much of their
sediment is carried far out to sea, accumulating on the continental
margins, and especially on the continental slopes. In deeper
ocean areas, the major sediment source are the organisms that
live near the ocean surface, whose skeletons, feces, and other
organic debris rain down slowly to the seafloor.
Not all of the seafloor has a sediment cover, however. In some
places, strong currents scour the seafloor clean, exposing the
bedrock below.
|
It is rare that nature produces anything
as a waste product for which some organism cannot find a use.
While methanogens simply dump the methane they produce, other
organisms live on it. Much of the free methane which eventually
finds its way up through the sediments is consumed by bacterial
or archaeal methanotrophs. Some bacterial methanotrophs are aerobic,
and therefore must live in the aerated (oxic) conditions at the
top of the ocean sediment or in the ocean itself. Within the deeper,
anoxic sediments, however, microorganisms frequently use sulfate
(ions containing both sulfur and oxygen) to provide energy, a
process known as sulfate-reduction.
Because sulfates is easily dissolved
in seawater, numerous sulfate-reducers are found in oceanic sediments.
(The amount of sulfate dissolved in seawater is truly extraordinary;
it has been estimated at some 1376 trillion metric tons: Brock
and Madigan, 1988, p. 630.) The higher concentrations of sulfate
are found close to the sediment surface, where organic carbon
debris, another essential foodstuff for sulfate-reducers, is also
found. There the sulfate-reducers congregate, living in consortia
with the methanotrophs, harvesting methane as it rises from below
(DeLong, 2000). In northern peatlands, the methanotrophs consume
as much as 90% of the methane available (Dedysh, 1998); it seems
likely that a similar or greater percentage of the methane escaping
from marine sediments may meet the same end.
Some methane undoubtedly makes it through
the gantlet of the methanotrophs in the topmost sediment, then
through the ocean itself and escapes into the atmosphere. Most
methane, however, never makes it even as far as the sediments
of the upper seafloor. Instead, as it rises through the deep sediments,
it quickly becomes trapped in lattice-like structures or cages
(called clathrates) composed of water ice. (Clathrates are microscopic
crystalline chemical structures. They are highly efficient storage
units and can contain several different kinds of gas, including
carbon dioxide.) At the proper conditions of temperature and pressure,
methane or other gases found in the porous sediments spontaneously
react with seawater to produce these structures.
Those clathrates which specifically hold
natural gas in their icy lattices are referred to as gas hydrates
("hydro" is the Greek word for water). There are three
gas hydrate structures, referred to as Structure I (sI), Structure
II (sII), and Structure H (sH). Structure I typically contains
exclusively methane; the other two structures are slightly larger
(though still microscopic) and can contain somewhat larger gas
molecules, though their primary constituent (called guest molecules)
remains methane. Structure I is the least stable, meaning that
it can break up ("dissociate") at lower pressures and
higher temperatures than the other structures. Structure II is
the most stable, while Structure H, previously known exclusively
from the laboratory and just recently found in the seafloor, is
intermediate in stability (Lu, 2007).
While other hydrocarbon gases are frequently
found together with methane in gas hydrates, the most common gas,
by far, is methane. It comprises more than 99% of the gas in gas
hydrates, with few other natural gases, like ethane, and occasionally
propane. Though the term "methane hydrate," rather than
gas hydrate is technically reserved for hydrates with greater
than 99.9% of their contents as methane, the terms herein will
be used interchangeably unless there is a specific reason to do
otherwise.
Methane hydrates are extremely efficient
in trapping methane, holding about 160 times the amount which
would be occupied by the equivalent volume of free methane. In
other words, a given volume of methane can be stored in a hydrate
only 1/160th its size. Consequently, methane hydrates can contain
huge quantities of methane.
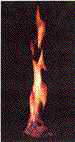
Methane hydrate, the "ice
that burns." As the methane
burns, the ice which formerly trapped it melts. (US Department
of Energy photo.)
|
Methane hydrates form wherever
methane and water are present at the proper temperature and pressure
conditions. In fact, an experiment in the Santa Barbara Basin
off southern California revealed that just as soon as methane
is released into deep, cold water, methane hydrates form. Because
methane hydrate is lighter than water, it quickly floats to the
surface. That is why chunks of methane hydrate as large as refrigerators
have been spotted floating on the ocean surface, where they rapidly
melt and their methane is released into the atmosphere. But because
methane hydrate typically forms deep within the seafloor sediments,
those sediments keep it in place. Consequently, despite the enormous
quantity of methane hydrate that exists, only small amounts are
found on the seafloor itself, where it can detach and float away,
or, as happened with the Canadian fishing boat, where it can
be scraped off the bottom itself. These chunks attract the curiosity
of those who find them because they can easily be set on fire,
burning off the methane and leaving just a puddle of water. Hence
the name, "the ice that burns." |
CONTINUE
TO NEXT SECTION (Methane and methane hydrates, Part 2)
RETURN
TO CONTENTS